A) II > I > III
B) I > II > III
C) III > I > II
D) III > II > I
F) C) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following is the strongest base?
A) CH3COCH3
B) CH3COOH
C) NH3
D) H2O
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the conjugate base of HSO4-? SO42- H2SO4 SO3 H2O I II III IV
A) I
B) II
C) III
D) IV
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the products of the following proton transfer reaction? 
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid? 
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the nucleophilic site in the following compounds? 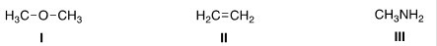
A) I = Hydrogen; II = π electrons in bond; III = nitrogen.
B) I = Oxygen; II = carbon; III = nitrogen.
C) I = Hydrogen; II = carbon; III = carbon.
D) I = Oxygen; II = π electrons in bond; III = nitrogen.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following will proceed as written?
A) CH3ONa + HCl → CH3OH + NaCl
B) CH3OH + NaCl → NaOEt + HCl
C) CH3OH + H2O → CH3O- + H3O+
D) CH3OH + NH3 → CH3O- + NH4±
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the role of methylchloride (CH3Cl) in the following reaction? 
A) Lewis acid
B) Lewis base
C) Brønsted-Lowry acid
D) Brønsted-Lowry base
F) None of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Identify the Lewis base in the following reaction. 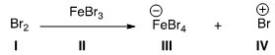
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is both a Brønsted-Lowry acid and base? 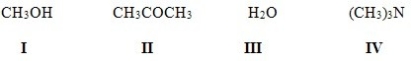
A) I,II
B) I,III
C) II,IV
D) I,IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct classification of the following compound? CH3-O-CH3
A) Brønsted-Lowry acid and Lewis acid
B) Brønsted-Lowry base and Lewis base
C) Brønsted-Lowry base
D) Lewis base
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the weakest acid?
A) H2S
B) PH3
C) HCl
D) SiH4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the direction of equilibrium when acetylene (C2H2) reacts with H2N- in an acid-base reaction? 
A) Left
B) Right
C) Neither
D) Cannot be determined
F) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following is a Lewis acid but not a Brønsted-Lowry acid?
A) CH3OH
B) H2O
C) CH3COOH
D) BF3
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the strongest acid?
A) CH3OH
B) BrCH2OH
C) CH3NH2
D) CH3Cl
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the conjugate acid in the following reaction? 
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct rank of the following compounds in order of increasing acidity? 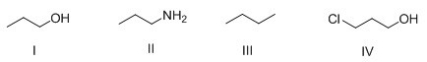
A) I > II > III > IV
B) IV > III > II > I
C) IV > I > II > III
D) III > I > IV > II
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the base/conjugate base (in that order) in the following reaction: 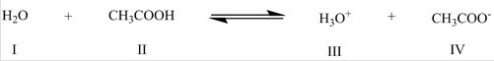
A) I,III
B) I,IV
C) II,III
D) II,IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species cannot act as both a Brønsted-Lowry acid and base?
A) HCO3-
B) HSO4-
C) HO-
D) H2PO4-
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of decreasing acidity,putting the most acidic first. 
A) IV > II > III > I
B) IV > III > II > I
C) III > IV > II > I
D) III > IV > I > II
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 59
Related Exams