A) CaN
B) CaN2
C) Ca2N
D) Ca3N2
E) Ca2N3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the Lewis structure of methanethiol,CH3SH?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many dots are present in the Lewis symbol for the fluorine atom?
A) 5
B) 6
C) 7
D) 8
E) 10
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
In the molecule BeF2,the beryllium atom is an exception to the octet rule.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula of the sulfate ion?
A) SO4-
B) SO3-
C) SO42-
D) SO32-
E) SO22-
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which formula represents ammonia?
A) Al3
B) AlH3
C) NH4+
D) NH3
E) AN4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the two principal classes of bonding called?
A) ionic bonding and nuclear bonding
B) covalent bonding and hydrogen bonding
C) hydrogen bonding and ionic bonding
D) polar bonding and ionic bonding
E) ionic bonding and covalent bonding
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
There are three atoms of iodine represented in the formula NaIO3.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following Lewis structures has a possible resonance structure? 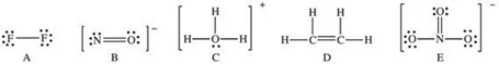
A) A
B) B
C) C
D) D
E) E
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds would be the most polar?
A) P-F
B) N-F
C) S-Cl
D) Br-Br
E) F-F
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Baking soda consists of the ionic compound sodium bicarbonate.What is the formula of this compound?
A) Na(CO3) 2
B) Na2CO3
C) NaBiCO3
D) NaHCO3
E) Na2HCO3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assuming reactions between the following pairs of elements,which pair is most likely to form a covalent compound?
A) lithium and iodine
B) sodium and oxygen
C) calcium and chlorine
D) copper and tin
E) carbon and oxygen
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the molecule AX2,the central atom A has two lone pairs of electrons in addition to the two bond pairs in the A-X bonds.What is the shape of this molecule?
A) bent,bond angle close to 109.5°
B) bent,bond angle close to 120°
C) linear
D) trigonal planar
E) trigonal pyramidal
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The drug "lithium" is often prescribed to treat mental illness.It is not the element lithium,but the ionic compound Li2CO3,that is actually administered.What is the name of this compound?
A) dilithium carbon trioxide
B) lithium carbide
C) lithium carboxide
D) lithium carbonate
E) lithium tricarbonate
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does it mean if an atom is said to have a high electronegativity?
A) The atom requires a large amount of energy to remove an electron from its structure.
B) The atom is highly susceptible to losing a valence electron.
C) The atom has a strong attraction for electrons in a chemical bond.
D) The atom releases a large amount of energy when it gains an electron.
E) The atom has more electrons around it than necessary; the atom has an expanded octet.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A double bond between two atoms,A and B,________.
A) is longer than a single bond between the same two atoms.
B) has a lower bond energy than a single bond between the same two atoms.
C) arises when two electrons are transferred from A to B.
D) consists of two electrons shared between A and B.
E) consists of four electrons shared between A and B.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula of the compound sulfur trioxide?
A) S3O
B) SO3
C) S(O2) 3
D) S3O2
E) S3O2?
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of Fe2(SO4) 3 in the Stock system?
A) iron monosulfuric acid
B) iron(II) sulfate
C) iron(III) sulfate
D) iron trisulfate
E) iron(II) trisulfate
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many bonding electrons are present in the Lewis structure for the bicarbonate ion,shown below? 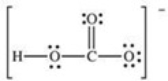
A) 4
B) 5
C) 8
D) 10
E) 24
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is wrong with the Lewis structure shown for sulfur trioxide,SO3? 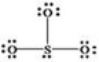
A) Oxygen,not sulfur,should be the central atom.
B) Sulfur should have 10 electrons around it instead of 8,to show its expanded octet.
C) The structure shows 26 valence electrons,but there should only be 24.
D) There are too many bonding electrons shown.
E) The structure should show each oxygen atom with a double bond to the sulfur atom.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 93
Related Exams