A) bent, bond angle close to 109.5o
B) bent, bond angle close to 120o
C) linear
D) trigonal planar
E) trigonal pyramidal
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound contains a central atom with an exception to the octet rule known as an expanded octet?
A) BeH2
B) NO
C) PF5
D) CH4
E) BF3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structures of F2, O2 and N2.Which statement is true?
A) All three molecules have a single bond between the atoms.
B) Both O2 and N2 have double bonds connecting the atoms.
C) Fluorine has the shortest bond between the two atoms.
D) The bond between the nitrogen atoms in N2 is stronger than the bond in F2 and O2.
E) All three molecules have triple bonds between the atoms.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule of deoxyribose, an essential part of DNA, contains five carbon atoms, ten hydrogen atoms, and four oxygen atoms.How would the formula of deoxyribose be represented?
A) 5C10H4O
B) C5H10O4
C) C5H10O4
D) C5H10O4
E) All formulas are acceptable.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many bonding electrons are in CO2?
A) 1
B) 2
C) 3
D) 4
E) 8
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the ion NH4+?
A) nitrogen tetrahydrate
B) nitrogen hydrate
C) mononitrogen tetrahydrogen
D) ninhydrin
E) ammonium
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about compounds is FALSE?
A) Compounds consist of atoms of two or more different elements that are chemically bonded together.
B) The elements in a compound cannot be separated or recovered by a physical process.
C) Ionic compounds are composed of cations and anions.
D) Covalent compounds are composed of metals and nonmetals.
E) Intermolecular forces are attractive forces between molecules of a compound, and are responsible for the compound?s physical properties.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What statement about the ammonia molecule, NH3, is FALSE?
A) The molecule contains three polar bonds.
B) The molecule itself is not polar.
C) The molecule has a trigonal pyramidal shape.
D) The nitrogen atom has one nonbonding pair of electrons.
E) The pull of the electrons in the N?H bonds is toward the nitrogen atom.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Because the C-H bond in methane is polar, the CH4 molecule will also be polar.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following Lewis structures represent resonance forms of ozone, O3? 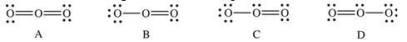
A) A and B
B) B and C
C) C and D
D) A and C
E) A and D
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vehicle airbags inflate when the ionic compound sodium azide rapidly decomposes to the elements sodium and nitrogen.If the azide ion is a polyatomic ion with the formula N3−, what is the formula of sodium azide?
A) NaN3
B) Na3N3
C) Na3(N3) 3
D) Na3N
E) Na(N3) 3
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 91 of 91
Related Exams