Filters
Question type
A) SO3
B) BF3
C) I3¯
D) SCO (C = central atom)
E) SO32¯
F) A) and B)
G) A) and D)
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Question 62
Multiple Choice
Select the correct Lewis structure for TeBr2.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
F) C) and D)
G) All of the above
G) All of the above
Correct Answer

verified
Correct Answer
verified
Question 63
Multiple Choice
Use VSEPR theory to decide which one of the following molecules and ions will definitely have at least one 90 bond angle in it. (In each case except water, the central atom is the first one in the formula.)
A) AlCl4¯
B) NH3
C) PCl5
D) CO2
E) H2O
F) None of the above
G) A) and B)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Question 64
True/False
All possible resonance structures contribute equally to the resonance hybrid.
A) True
B) False
B) False
Correct Answer

verified
Correct Answer
verified
Question 65
Multiple Choice
What is the molecular shape of ClO3F as predicted by the VSEPR theory? 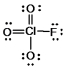
A) trigonal pyramidal
B) square planar
C) square pyramidal
D) tetrahedral
E) octahedral
F) C) and D)
G) A) and D)
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Question 66
Essay
Draw Lewis structures which obey the octet rule, for the following atoms, molecules and ions, showing all valence electrons. Central atoms are shown in bold. A) NH3 b. O3 (Hint: O3 is not cyclic) C) HCN D) SO3
Correct Answer

verified
Correct Answer
verified
Question 67
True/False
A molecule which contains polar bonds will always have a dipole moment.
A) True
B) False
B) False
Correct Answer

verified
Correct Answer
verified
Question 68
Multiple Choice
How many resonance structures are possible for NO3¯?
A) 1
B) 2
C) 3
D) 4
E) 5
F) D) and E)
G) C) and D)
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 108 of 108
Related Exams