A) NH 3
B) NH 4 +
C) I 2
D) BH 4 −
E) SF 6
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best Lewis structure for P2I4.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosphoryl iodide is used in the preparation of organophosphorus derivatives and phosphate esters. Select the Lewis structure for POI3 that minimizes formal charges.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
C
Correct Answer
verified
True/False
Boron never achieves an octet in any of its compounds.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many resonance structures are possible for NO3−?
A) 1
B) 2
C) 3
D) 4
E) 5
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules does not have a dipole moment?
A) CS 2
B) H 2S
C) CH 2Cl 2
D) PH 3
E) CH 2O
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
In order for a noncyclic triatomic molecule to be bent, VSEPR theory requires that there must be two lone pairs on the central atom.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles in GeCl4 using the molecular shape given by the VSEPR theory.
A) 90°
B) 109°
C) 120°
D) 180°
E) < 90°
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct Lewis structure for NOCl, a reactive material used as an ionizing solvent.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following Lewis structure for phosphate, phosphorus has a formal charge of __________ and an oxidation number of __________. 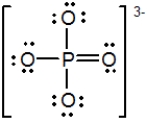
A) 0; −3
B) 0; 5
C) 5; −3
D) 5; 5
E) 3; 5
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules has a zero dipole moment?
A) SO 2
B) HCl
C) CS 2
D) CO
E) Cl 2O
G) B) and E)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following molecules has a net dipole moment?
A) BeCl 2
B) SF 2
C) KrF 2
D) CO 2
E) CCl 4
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of NH2Cl as predicted by the VSEPR theory?
A) Trigonal pyramidal
B) Tetrahedral
C) T-shaped
D) See-saw
E) Trigonal planar
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The molecule AX2, where A and X are different elements, will have a dipole moment if the molecule is bent.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
All possible resonance structures contribute equally to the resonance hybrid.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The Lewis structure of NO2 violates the octet rule.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
When resonance occurs, the bond lengths in a molecule fluctuate rapidly.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules contains a double bond?
A) N 2
B) PCl 5
C) CH 2O
D) C 2H 2
E) I 2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the Lewis structure for XeO2F2 that correctly minimizes formal charges.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following Lewis structure for ClO3F, chlorine has a formal charge of __________ and an oxidation number of __________. 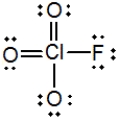
A) 7; 7
B) 7; −1
C) 1; 1
D) 1; −1
E) 1; 7
G) A) and B)
Correct Answer

verified
E
Correct Answer
verified
Showing 1 - 20 of 98
Related Exams