Correct Answer

verified
Correct Answer
verified
Short Answer
Use one the following terms to complete the given statement. salt sugar vegetable oil When ____________________ is added to water a heterogeneous mixture forms.
Correct Answer

verified
Correct Answer
verified
Short Answer
Fill in each blank with the appropriate term from the list given below. protons neutrons electrons valence electrons -14N and 15N have the same number of _____________________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass of one mole of glucose, C6H12O6?
A) 29.02 amu
B) 180.2 amu
C) 29.02 g
D) 180.2 g
F) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The elements sodium, potassium, and oxygen are considered to be "elements of life" and are in the category of electrolytes.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly describes a proton on a subatomic scale?
A) It is massive and has a +1 charge.
B) It is massive and has a -1 charge.
C) It has very small mass and a +1 charge.
D) It has a very small mass and a -1 charge.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
An element has the following electron arrangement. shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 7 electrons
The Lewis structure for this element would be: 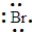
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Elements in the same period of the periodic table generally show similar chemical behavior.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Group 4A and Group 14 are two different designations for the same column of the periodic table.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Enter the chemical symbol of the element in the blank. Calcium forms the compound CaF2 . What other alkaline earth element with a smaller atomic mass would form a compound with a similar formula? Chemical symbol:_____________________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula weight of ibuprofen, C13H18O2?
A) 29.0 g/mol
B) 206.3 g/mol
C) 289.4 g/mol
D) 377.7 g/mol
F) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
An isotope of gallium consisting of 31 protons and 37 neutrons can be represented using the symbol shown below. gallium-37
B) False
Correct Answer

verified
Correct Answer
verified
True/False
An element is a shiny gray solid that can be pressed into a thin sheet. This element is probably a metalloid.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct unit for the formula weight of a large number of atoms?
A) amu
B) g
C) mol
D) molecules
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sealed cylinder is filled with a large collection of atoms that have 14 neutrons and 13 protons. Which of the following would behave in a manner similar to this collection of atoms?
A) a group of atoms with 13 neutrons and 14 protons
B) a group of atoms with 14 neutrons and 15 protons
C) a group of atoms with 15 neutrons and 14 protons
D) a group of atoms with 15 neutrons and 13 protons
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Enter an integer number (1, 2, 3, ...) in the blank. Sucrose (table sugar) has the formula C12H22O11. A sample of sucrose contains 2400 carbon atoms. How many formula units does this represent? ____________________formula units.
Correct Answer

verified
Correct Answer
verified
Short Answer
Enter an integer number (1, 2, 3, ...) in the blank. How many electrons are in shell 2 of a P atom? ____________________electrons
Correct Answer

verified
Correct Answer
verified
Showing 61 - 77 of 77
Related Exams