A) a system becomes warmer and the chemical substances undergo an increase in potential energy.
B) a system becomes warmer and the chemical substances undergo a decrease in potential energy.
C) a system becomes cooler and the chemical substances undergo an increase in potential energy.
D) a system becomes cooler and the chemical substances undergo a decrease in potential energy.
E) a system becomes warmer and additional heat is gained from the surroundings.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 25.00 gram pellet of lead (specific heat = 0.128 J g-1 °C-1) at 25 °C is added to 95.3 g of boiling water (specific heat of 4.18 J g-1 °C-1) at 100 °C in an insulated cup. What is the expected final temperature of the water?
A) 26.6 °C
B) 62.5 °C
C) 84.4 °C
D) 99.4 °C
E) 100.6 °C
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A closed, uninsulated system was fitted with a movable piston. Introduction of 483 J of heat caused the system to expand, doing 320 J of work in the process against a constant pressure of 101 kPa (kilopascals) . What is the value of H for this process?
A) (483 + 320) joules
B) (483 - 320) joules
C) (320 - 483) joules
D) 483 joules
E) (-320 - 483) joules
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard enthalpy of combustion for xylene, C8H10(l) , is -3908 kJ mol-1. Using this information and the standard enthalpies of formation of the following, H°f:H2O(l) = -285.9 kJ mol-1; CO2(g) = -393.5 kJ mol-1, calculate the standard enthalpy of formation of C8H10(l) , in kJ mol-1.Hint: To begin, determine the thermochemical equation associated with the standard enthalpy for the formation of xylene.
A) -669.5 kJ
B) +3228.6 kJ
C) -3228.6 kJ
D) +4587.4 kJ
E) +8485.5 kJ
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the thermochemical equation, 3 M(s)+ 3 O2(g)→ 3 MO2(s)with a standard enthalpy of reaction = −1443 kJ, calculate the value for ΔH°reaction for the reaction:MO2(s)→ M(s)+ O2(g).
Correct Answer

verified
Correct Answer
verified
Short Answer
For a chemical reaction in which the change is endothermic, the sign of the enthalpy change, H, is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the standard enthalpy change, H°, for the reaction, CCl4(l) + 4HCl(g) CH4(g) + 4Cl2(g) , given the following thermochemical equations: 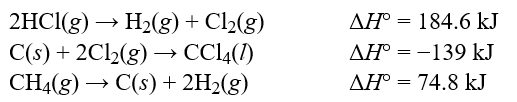 Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
Hint: Pay careful attention to your signs. If you reverse an equation remember to change the sign appropriately.
A) +55.3 kJ
B) -187 kJ
C) +101 kJ
D) -179 kJ
E) +433 kJ
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is a unit of energy, but is not the SI unit of energy?
A) joule
B) newton
C) pascal
D) watt
E) calorie
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A constant pressure calorimeter has metal parts (heat capacity of 925.0 J °C-1) and 1.100 × 103 grams of oil (specific heat = 2.824 J g-1 °C-1) , both at 25.40°C. Adding a 5.50 × 102 g slug at 220.0°C, caused the temperature to rise to 35.2 °C. Find the specific heat of the metal.Hint: Consider the amount of energy that was required to raise the temperature of the entire system.
A) 0.365 J g-1 °C-1
B) 0.389 J g-1 °C-1
C) 0.395 J g-1 °C-1
D) 0.551 J g-1 °C-1
E) 1.20 J g-1 °C-1
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 0.250 moles of LiCl are added to 200.0 g of water in a constant pressure calorimeter a temperature change of +11.08 °C is observed. Given that the specific heat of the resulting solution is 4.184 J g-1 °C and we can ignore the small amount of energy absorbed by the calorimeter, what is the molar enthalpy of solution ( Hsol) for LiCl?Hint: Do not ignore the specific heat of water in this problem.
A) 37.1 kJ/mol
B) -185.4 kJ/mol
C) -37.1 kJ/mol
D) 18.5 kJ/mol
E) -18.5 kJ/mol
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
When the Kelvin temperature of a sample of molecules increases, its average kinetic energy ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the enthalpy change, ?H, for the reaction, N2(g) + 2H2(g) N2H4(l) , given the following thermochemical equations: 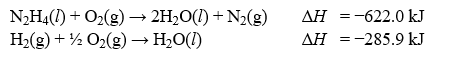
A) -151.7 kJ
B) -236.2 kJ
C) +106.1 kJ
D) +50.2 kJ
E) +567.4 kJ
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The thermochemical equation that is associated with H°f, the standard enthalpy of formation for glucose, C6H12O6(s) , is
A) 6 C(s) + 6 H2O(l) C6H12O6(s) .
B) 6 C(s) + 12 H(g) + 6 O(g) C6H12O6(s) .
C) 6 C(s) + 6 H2(g) + 3 O2(g) C6H12O6(s) .
D) 2 C2H5OH(l) + 2 CO2(g) C6H12O6(s) .
E) 6 C(g) + 6 H2(g) + 3 O2(g) C6H12O6(s) .
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical reaction took place in a 5 liter cylindrical enclosure fitted with a piston (like the cylinder in an internal combustion engine) . Over the time required for the reaction to be completed, the volume of the system changed from 1.40 liters to 3.70 liters. Which of the following statements below is true?
A) The enthalpy of the system remained unchanged.
B) The enthalpy of the system decreased.
C) The enthalpy of the system increased.
D) Work was performed by the system.
E) Work was performed on the system.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 500.0 gram sample of aluminum is initially at 25.0 °C. It absorbs 32.60 kJ of heat from its surroundings. What is its final temperature, in °C? (specific heat = 0.9930 J g-1 °C-1 for aluminum)
A) 40.4 °C
B) 64.7 °C
C) 65.7 °C
D) 89.7 °C
E) 90.7 °C
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The temperature change, measured in Kelvins, experienced by an object, is directly proportional to the heat it absorbs.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is a unit of energy?
A) pascal
B) newton
C) joule
D) watt
E) ampere
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the enthalpy change, H, for the reaction, W(s) + C(s) ? WC(s) , given the following thermochemical equations: 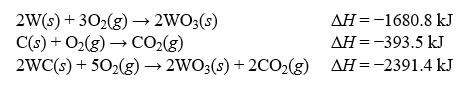
A) +33.3 kJ
B) -38.2 kJ
C) +106.1 kJ
D) -52.9 kJ
E) +177.4 kJ
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 55.00 gram pellet of lead at 25 °C is added to 58.5 g of boiling water (specific heat of 4.18 J g-1 °C-1) at 100 °C in an insulated cup. If the final temperature of the water in the cup is97.9 °C, what is the specific heat of lead?
A) 17.8 J g-1 °C-1
B) 0.128 J g-1 °C-1
C) 4.17 J g-1 °C-1
D) 22.2 J g-1 °C-1
E) 0.372 J g-1 °C-1
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The standard enthalpy of combustion for oxalic acid, H2C2O4(s), is −251.9 kJ mol−1. Using this data and the standard enthalpies of formation, 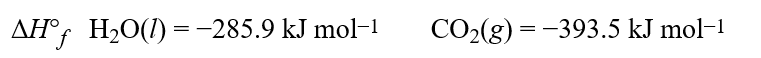 calculate the standard enthalpy of formation of H2C2O4(s), in kJ mol−1.Hint: Pay careful attention to your signs when calculating the enthalpy.
calculate the standard enthalpy of formation of H2C2O4(s), in kJ mol−1.Hint: Pay careful attention to your signs when calculating the enthalpy.
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 176
Related Exams