A) ClO-
B) Cl-
C) ClO2-
D) Cl2
E) ClO3-
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
When a copper metal reacts with aqueous nitric acid, which substance is the oxidizing agent?
Correct Answer

verified
Correct Answer
verified
Short Answer
When H2O2 behaves as a reducing agent, the most likely product formed from it is ________. Hint: Write out an example equation with H2O2 being oxidized to see the result.
Correct Answer

verified
Correct Answer
verified
Short Answer
Balance the redox reaction below in basic solution.PbO2(s)+ I-(aq)→ Pb2+(aq)+ I2(s)What is the coefficient of OH- in the final balanced equation? Hint: Consider your oxidation numbers when balancing redox reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The CrO42- ion was involved in a chemical reaction, during which it is transformed into Cr3+ ions. What is the change in oxidation number of the chromium atom?
A) The oxidation number increases by 5 units.
B) The oxidation number decreases by 5 units.
C) The oxidation number increases by 1 unit.
D) The oxidation number decreases by 3 units.
E) The oxidation number decrease by 4 units.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Balance the redox reaction below in acidic solution.Cl2(g)→ Cl-(aq)+ ClO-(aq)When balanced, what is the sum of all the coefficients? Hint: Consider your oxidation numbers when balancing redox reactions.
Correct Answer

verified
Correct Answer
verified
True/False
The reaction, AgNO3(aq)+ NH4Br(aq) AgBr(s)+ NH4NO3(aq), involves changes in oxidation number and is therefore classified as a redox reaction.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the balancing of the following half-reaction, taking place in basic media. How many hydroxide ions are needed to balance the half-reaction?Br(aq) s BrO3(aq) Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A) 2 hydroxide ions, on the left side
B) 4 hydroxide ions, on the left side
C) 4 hydroxide ions, on the right side
D) 6 hydroxide ions, on the left side
E) 6 hydroxide ions, on the right side
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
When the equation, Al(s)+ NO3-(aq)→ NO(g)+ Al3+(aq)is balanced for an acidic solution, the sum of ALL the coefficients is ________. Hint: Do not neglect to include electrons when balancing redox reactions.
Correct Answer

verified
Correct Answer
verified
True/False
In order for an oxidation reaction to occur, a reduction reaction must also take place.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balance the half-reaction, C2H6O HC2H3O2, taking place in acidic media. How many electrons are needed to balance the charge?Hint: Remember the coefficients when balancing half-reactions and look carefully at the oxidation numbers.
A) 2 electrons, left side
B) 3 electrons, left side
C) 4 electrons, right side
D) 6 electrons, right side
E) 8 electrons, right side
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The following chemical equation shows how water can be produced. H2(g)+ ½ O2(g) H2O(l)In the reaction, hydrogen is the species which is reduced.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most reactive metals are those of
A) Group IA (Group 1) .
B) Group IIA (Group 2) .
C) Group IIIA (Group 13) .
D) Group IB (Group 11) .
E) Group IIB (Group 12) .
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nitrogen molecule, (N2) , was involved in a chemical reaction in which it underwent reduction. Based on the change in oxidation numbers, which of the species listed below is a possible product of the reaction?
A) N2H4
B) NO
C) NO2-
D) NO2
E) NO3-
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
A partial activity series is: Au < Pt < Ag < Cu < H2 < Sn < Co < Fe < Mg. Based on this series, which of the following solutions would cause a reaction with Cu(s)? 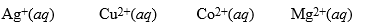
Correct Answer

verified
Correct Answer
verified
True/False
Hot concentrated sulfuric acid is a good oxidizing agent.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following involves oxidation?
A) Ca2+(aq) + CO32-(aq) CaCO3(s)
B) 2 H+(aq) + SO32-(aq) H2O(l) + SO2(g)
C) VO43-(aq) VO2+(aq)
D) CrO2- (aq) CrO42-(aq)
E) 2 S2O72-(aq) + H2O(l) 2 SO42-(aq) + 2 H+(aq)
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The following reaction describes the decomposition of NaHCO3, baking soda:2NaHCO3 Na2CO3 + CO2 + H2O This is not an oxidation-reduction reaction, since nothing is oxidized or reduced.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following involves reduction?
A) Ba2+(aq) + CrO42-(aq) BaCrO4(s)
B) 2 H+(aq) + CO32-(aq) H2O(l) + CO2(g)
C) CrO42-(aq) Cr3+(aq)
D) MnO2(s) MnO4-(aq)
E) 2 CrO42-(aq) + 2 H+(aq) Cr2O72-(aq) + H2O(l)
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Zinc metal reacts with perchloric acid solution to produce aqueous zinc perchlorate and hydrogen gas, which escapes. The species being oxidized in this reaction is
A) HClO4(aq) .
B) H2(g) .
C) Zn2+(aq) .
D) Zn(s) .
E) Zn(ClO4) 2(aq) .
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 175
Related Exams