Correct Answer

verified
Correct Answer
verified
True/False
The equilibrium between carbon dioxide gas and carbonic acid is very important in biology and environmental science, and is shown below.
CO2(aq)+ H2O(l)
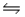 H2CO3(aq)
Based on the equilibrium constant reported for this reaction (Kc = 1.70 × 10-3), there will be more carbon dioxide in solution than carbonic acid.
H2CO3(aq)
Based on the equilibrium constant reported for this reaction (Kc = 1.70 × 10-3), there will be more carbon dioxide in solution than carbonic acid.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction at equilibrium in a reaction vessel, which change will cause the Br2 concentration to decrease? 2NOBr(g) 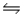 2NO(g) + Br2(g) , Hºrxn= 30 kJ/mol
2NO(g) + Br2(g) , Hºrxn= 30 kJ/mol
A) Increase the temperature.
B) Remove some NO.
C) Add more NOBr.
D) Compress the gas mixture into a smaller volume.
E) Add a catalyst
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The brown gas NO2 and the colorless gas N2O4 exist in equilibrium, 2NO2 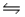 N2O4.In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached.The concentration of N2O4 at equilibrium was 0.0750 M.Calculate Kc for the reaction.
N2O4.In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached.The concentration of N2O4 at equilibrium was 0.0750 M.Calculate Kc for the reaction.
A) 7.5
B) 0.125
C) 0.0750
D) 0.10
E) 0.050
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction H2(g) + I2(g) ![For the reaction H<sub>2</sub>(g) + I<sub>2</sub>(g) <sub> </sub> 2HI(g) , K<sub>c</sub> = 50.2 at 445ºC.If [H<sub>2</sub>] = [I<sub>2</sub>] = [HI] = 1.75 × 10<sup>-3</sup> M at 445ºC.Which of the following is true based on the above? A) Q<sub>c</sub> > K<sub>c</sub>, the reaction proceeds from left to right to reach equilibrium B) Q<sub>c</sub> > K<sub>c</sub>, the reaction proceeds from right to left to reach equilibrium C) Q<sub>c</sub> < K<sub>c</sub>, the reaction proceeds from left to right to reach equilibrium D) Q<sub>c</sub> < K<sub>c</sub>, the reaction proceeds from right to left to reach equilibrium E) Q<sub>c</sub> = K<sub>c</sub>, the reaction is currently at equilibrium](https://d2lvgg3v3hfg70.cloudfront.net/TB3246/11ea7cbf_8f1e_ceb1_a2ab_e557c292b501_TB3246_11.jpg) 2HI(g) , Kc = 50.2 at 445ºC.If [H2] = [I2] = [HI] = 1.75 × 10-3 M at 445ºC.Which of the following is true based on the above?
2HI(g) , Kc = 50.2 at 445ºC.If [H2] = [I2] = [HI] = 1.75 × 10-3 M at 445ºC.Which of the following is true based on the above?
A) Qc > Kc, the reaction proceeds from left to right to reach equilibrium
B) Qc > Kc, the reaction proceeds from right to left to reach equilibrium
C) Qc < Kc, the reaction proceeds from left to right to reach equilibrium
D) Qc < Kc, the reaction proceeds from right to left to reach equilibrium
E) Qc = Kc, the reaction is currently at equilibrium
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the equilibrium reaction 2SO2(g) + O2(g) 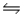 2SO3(g) , Hºrxn = -198 kJ/mol.Which one of these factors would cause the equilibrium constant to increase?
2SO3(g) , Hºrxn = -198 kJ/mol.Which one of these factors would cause the equilibrium constant to increase?
A) Decrease the temperature.
B) Add SO2 gas.
C) Remove O2 gas.
D) Add a catalyst.
E) None of these.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
When the reaction 2O3(g) 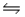 3O2(g), for which Kp = 3.0 × 1026 at 773ºC, is at equilibrium, the mixture will contain very little O2 as compared to O3.
3O2(g), for which Kp = 3.0 × 1026 at 773ºC, is at equilibrium, the mixture will contain very little O2 as compared to O3.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction N2(g) + O2(g) 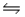 2NO(g) , for which Kc = 0.10 at 2,000ºC.Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
2NO(g) , for which Kc = 0.10 at 2,000ºC.Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
A) 5.4 × 10-3 M
B) 0.0096 M
C) 0.013 M
D) 0.080 M
E) 0.10 M
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the chemical reaction 2NH3(g) 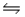 N2(g)+ 3H2(g).The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 × 10-2.Initially, 1,220 moles of NH3(g)are present.Estimate the equilibrium concentration of N2(g).
N2(g)+ 3H2(g).The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 × 10-2.Initially, 1,220 moles of NH3(g)are present.Estimate the equilibrium concentration of N2(g).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 250ºC, the equilibrium constant Kp for the reaction PCl5(g) 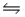 PCl3(g) + Cl2(g) is 1.80.Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC.Calculate the pressure of PCl5 after the system has reached equilibrium.
PCl3(g) + Cl2(g) is 1.80.Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC.Calculate the pressure of PCl5 after the system has reached equilibrium.
A) 1.50 atm
B) 1.24 atm
C) 4.24 atm
D) 0.94 atm
E) 1.12 atm
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reactions and their associated equilibrium constants: 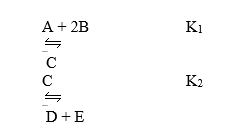 For the reaction A + 2B
For the reaction A + 2B 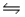 D + E, having equilibrium constant Kc,
D + E, having equilibrium constant Kc,
A) Kc = K1 + K2
B) Kc = K1/K2
C) Kc = K1 - K2
D) Kc = (K1) (K2)
E) Kc = K2/K1
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction at equilibrium, which gives a change that will shift the position of equilibrium to favor formation of more products? 2NOBr(g) 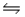 2NO(g) + Br2(g) , Hºrxn = 30 kJ/mol
2NO(g) + Br2(g) , Hºrxn = 30 kJ/mol
A) Increase the total pressure by decreasing the volume.
B) Add more NO.
C) Remove Br2.
D) Lower the temperature.
E) Remove NOBr selectively.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Kp for the reaction 4CuO(s) 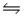 2Cu2O(s) + O2(g) is 0.49 at 1024 °C.Calculate Kc at this temperature
2Cu2O(s) + O2(g) is 0.49 at 1024 °C.Calculate Kc at this temperature
A) 5.8 x 10-3
B) 41
C) 52
D) 4.6 x 10-3
E) 0.49
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the equilibrium C(s)+ H2O(g) 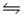 CO(g)+ H2(g), H = 2296 J.What will happen to the concentration of carbon if gaseous water is added to the system?
CO(g)+ H2(g), H = 2296 J.What will happen to the concentration of carbon if gaseous water is added to the system?
Correct Answer

verified
Correct Answer
verified
Short Answer
A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively.These ions react according to the following chemical equation
 What will be the concentration of Cu2+(aq)at equilibrium?
What will be the concentration of Cu2+(aq)at equilibrium?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction SO2(g) + NO2(g) 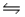 SO3(g) + NO(g) , the equilibrium constant is 18.0 at 1,200ºC.If 1.0 mole of SO2 and 2.0 moles of NO2 are placed in a 20.L container, what concentration of SO3 will be present at equilibrium?
SO3(g) + NO(g) , the equilibrium constant is 18.0 at 1,200ºC.If 1.0 mole of SO2 and 2.0 moles of NO2 are placed in a 20.L container, what concentration of SO3 will be present at equilibrium?
A) 0.48 mol/L
B) 0.11 mol/L
C) 0.95 mol/L
D) 2.22 mol/L
E) 18 mol/L
G) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
The data below refer to the following reaction:
2NO(g)+ Br2(g) 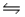 2NOBr(g)
2NOBr(g)  Calculate Kc.
Calculate Kc.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these situations will result if some CH4(g) is removed from the reaction CO(g) + 3H2(g) 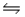 CH4(g) + H2O(g) at equilibrium?
CH4(g) + H2O(g) at equilibrium?
A) H2O will be consumed.
B) More CH4 and H2O will be produced.
C) Kp will decrease.
D) More CO will be produced.
E) No change will occur.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate Kp for the reaction 2NOCl(g) 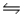 2NO(g) + Cl2(g) at 400°C if Kc at 400°C for this reaction is 2.1 × 10-2.
2NO(g) + Cl2(g) at 400°C if Kc at 400°C for this reaction is 2.1 × 10-2.
A) 2.1 × 10-2
B) 1.7 × 10-3
C) 0.70
D) 1.2
E) 3.8 × 10-4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Equilibrium is established for the reaction 2X(s) + Y(g) 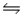 2Z(g) at 500K, Kc = 100.Determine the concentration of Z in equilibrium with 0.2 mol X and 0.50 M Y at 500K.
2Z(g) at 500K, Kc = 100.Determine the concentration of Z in equilibrium with 0.2 mol X and 0.50 M Y at 500K.
A) 3.2 M
B) 3.5 M
C) 4.5 M
D) 7.1 M
E) None of these.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 109
Related Exams