A) the loss of electrons
B) the reaction with oxygen
C) the reduction of oxygen
D) the gaining of electrons
E) the addition of an electron to the valence shell
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is it easier for the body to excrete a polar molecule than it is to excrete a nonpolar molecule? What chemistry does the body use to get rid of molecules it no longer needs?
A) It is easier for the body to excrete non-polar molecules because they dissolve in the oils of the skin and are washed away when we bathe.
B) Polar molecules are easier to excrete because of their greater solubility in water. Upon dissolving in water, they can be excreted through urine.
C) Polarity of the molecules has nothing to do with the ease of excretion. However, the body does metabolize and excrete smaller molecules more easily and passes them out through the colon.
D) Polar and non-polar molecules are excreted equally easily. The body is well adapted to deal with both forms of molecules via its metabolic processes.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does an atom's electronegativity relate to its ability to become oxidized?
A) The greater the electronegativity of an atom, the greater its ability to become oxidized.
B) The lower the electronegativity of an atom, the lower its ability to become oxidized.
C) The greater the electronegativity of an atom, the lower its ability to become oxidized.
D) Electronegativity does not effect the atom's ability to become oxidized.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is the junction barrier between n-type and p-type silicon much like a one-way valve?
A) The electrons can only be lost from the p-type silicon wafer.
B) The orientation of the charge around the barrier allows the electrons to flow in only one direction.
C) The electrons can only be lost from the n-type silicon wafer.
D) Only one side of the barrier is exposed to light, which causes the electrons to flow in the opposite direction.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Glucose, 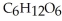 , is a simple sugar that the body metabolizes into two molecules of pyruvic acid,
, is a simple sugar that the body metabolizes into two molecules of pyruvic acid, 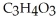 . Is the glucose oxidized or reduced as it transforms into pyruvic acid?
. Is the glucose oxidized or reduced as it transforms into pyruvic acid?
A) The glucose is oxidized.
B) The glucose is reduced.
C) Parts of the glucose molecule are oxidized while others are reduced.
D) Glucose is neither oxidized or reduced as it transforms into pyruvic acid.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an operating photovoltaic cell, electrons move through the external circuit to the negatively charged p-type silicon wafer. How can the electrons move to the negatively charged silicon when they themselves are negatively charged? Don't like signs repel each other?
A) This is a technological hurdle that has greatly inhibited the advancement of photovoltaic technology.
B) An electric current is possible because the energy from the sun momentarily reverses the charge of the electrons, which are then called positrons (p-type) .
C) The p-type silicon wafer is actually positively charged.
D) The energy of the sunlight knocks the electrons into a direction that they would otherwise not go.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A battery operates by ________.
A) oxidation
B) reduction
C) both oxidation and reduction
D) none of the above
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements describes what happens when you press a n-type silicon wafer against a p-type silicon wafer?
A) The n-type wafer becomes slightly positive, while the p-type becomes slightly negative.
B) The p-type wafer becomes slightly positive, while the n-type becomes slightly negative.
C) There is no change.
D) They both become slightly negative.
E) They both become slightly positive.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What might the relationship be between an element's ionization energy and its ability to behave as an oxidizing agent?
A) As the ionization energy increases, the ability of an element to act as an oxidizing agent increases.
B) As the ionization energy increases, the ability of an element to act as an oxidizing agent decreases.
C) As the ionization energy increases, the ability of an element to act as an oxidizing agent stays the same.
D) none of the above
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the primary difference between a fuel cell and a battery?
A) Fuel cells do not run down because they can be refueled; batteries run down and need to be recharged.
B) Batteries can be recharged, fuel cells cannot.
C) Batteries supply electricity; fuel cells supply heat.
D) Fuel cells oxidize to supply electricity, batteries reduce to supply electricity.
E) Fuel cells do not use metals as oxidants and reductants, batteries have a static reservoir of oxidant and reductant.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A major source of chlorine gas, 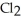 , is from the electrolysis of brine, which is concentrated salt water,
, is from the electrolysis of brine, which is concentrated salt water, 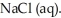 Which of the following is the balanced chemical reaction for this electrolysis reaction?
Which of the following is the balanced chemical reaction for this electrolysis reaction?
A) 2 NaCl (aq) + 2 H2O → 2 NaOH (aq) + ![]() (g) + 2
(g) + 2 ![]() (g)
(g)
B) 2 NaCl (aq) + ![]() O →
O → ![]() O (aq) + 2 HCl (aq)
O (aq) + 2 HCl (aq)
C) 2 NaCl (aq) + 2 ![]() O → 2 Na (s) + O2 (g) + 2
O → 2 Na (s) + O2 (g) + 2 ![]() (g) +
(g) + ![]() (g)
(g)
D) 2 NaCl (aq) + ![]() O → 2 NaH (aq) +
O → 2 NaH (aq) + ![]() O⁻ (aq)
O⁻ (aq)
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Upon combustion, about how many grams of water vapor are produced from every 16 grams of methane, 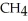 ?
?
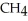 + 2
+ 2 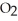 →
→ 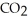 + 2
+ 2 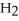 O
O
A) 16 grams
B) 18 grams
C) 36 grams
D) 8 grams
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chemical equations need to be balanced not only in terms of the number of atoms, but also by the charge. In other words, just as there should be the same number of atoms before and after the arrow of an equation, there should be the same charge. What set of coefficients is necessary to balance the following chemical equation? ____ 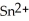 + ____ Ag → ____ Sn + ____ Ag⁺
+ ____ Ag → ____ Sn + ____ Ag⁺
A) 1, 2, 1, 2
B) 1, 1, 1, 2
C) 1, 2, 2, 2
D) 1, 1, 2, 1
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the half-reactions below would be complementary, balance the following half-reaction, and be chemically reasonable? Br2 → 2 Br-
A) 2 K → 2 K+
B) K+ → K
C) K → K+
D) 2 K+ → 2 K
E) K → K2+
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What might the relationship be between an element's electronegativity and its ability to behave as an oxidizing agent?
A) As the electronegativity increases the ability of an element to act as an oxidizing agent increases.
B) As the electronegativity increases the ability of an element to act as an oxidizing agent decreases.
C) As the electronegativity increases the ability of an element to act as an oxidizing agent stays the same.
D) none of the above
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The elements that make the best reducing agents are the ________.
A) group 18 noble gases
B) group 17 halogens
C) transition metals
D) group 1 alkali metals
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element is closer to the upper right corner of the periodic table, the one indicated by the lighter colored atoms or the darker colored atoms? 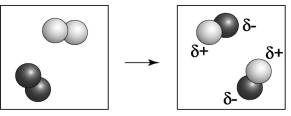
A) the element indicated by the darker color
B) the element indicated by the lighter color
C) More information is needed in order to determine which element is closer to the upper right of the periodic table.
D) Both the lighter and darker colored elements are likely found at the upper right corner of the periodic table.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iron atoms have a greater tendency to oxidize than do copper atoms. Is this good news or bad news for a home in which much of the plumbing consists of iron and copper pipes connected together? Explain.
A) This is bad news, since the iron atoms will be reduced by the copper atoms.
B) This is not a problem, since the strength of the copper will make up for the weakness of the iron.
C) It is bad news. The iron atoms will lose electrons to the copper atoms, which will pass those electrons onto oxygen atoms that are in contact with the surface.
D) This is not a problem since any electrons lost will be replaced by copper electrons.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is one of the advantages of a fuel cell?
A) They can run for a very long time as long as you keep adding fuel.
B) They are more efficient than combustion.
C) They have lower emission of pollutants than combustion.
D) all of the above
E) only A and B
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chemical equations need to be balanced not only in terms of the number of atoms, but also by the charge. In other words, just as there should be the same number of atoms before and after the arrow of an equation, there should be the same charge. What set of coefficients is necessary to balance the following chemical equation? ____ 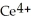 + ____ Cl⁻ → ____
+ ____ Cl⁻ → ____ 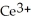 + ____
+ ____ 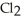
A) 1, 2, 1, 1
B) 2, 2, 2, 1
C) 1, 4, 1, 2
D) 3, 4, 3, 2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 138
Related Exams