A) CH3CH2CH2CH2NH2
B) (CH3) 3N
C) pyridine
D) (CH3) 3CNH2
E) none of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
What reagent can be used to convert benzophenone into triphenylmethanol?
Correct Answer

verified
PhMgBr followed by hydrolysis
Correct Answer
verified
Multiple Choice
Give the product for the following reaction. 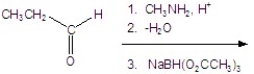
A) CH3CH2NHCH3
B) CH3CH2CH2OH
C) ![]()
D) CH3CH2CH2NHCH3
E) CH3CH2CH2N(CH3) 2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Propose a synthesis of 3-methyl-4-heptyn-3-ol from 1-butyne.
Correct Answer

verified
1) NaNH2
2)...View Answer
Show Answer
Correct Answer
verified
2)...
View Answer
Essay
By which single-step process can benzene be readily converted into acetophenone?
Correct Answer

verified
CH3COCl, AlCl3 - a Friedel-Crafts acylation reaction
Correct Answer
verified
Essay
Provide a detailed, stepwise mechanism for the acid-catalyzed condensation reaction between benzaldehyde and methylamine.
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of the hydrate that results when 4-heptanone is treated with dilute aqueous acid.
Correct Answer

verified
Correct Answer
verified
Essay
Give the product of the following reaction. 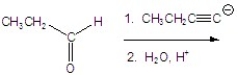
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why do aldehydes undergo nucleophilic addition reactions while esters undergo nucleophilic acyl substitution reactions?
A) The carbonyl carbon of an ester is more electrophilic than that of an aldehyde.
B) Aldehydes are more sterically hindered than esters.
C) Once the nucleophile adds to an aldehyde, the tetrahedral intermediate is too sterically hindered to eliminate one of the attached groups.
D) The ester carbonyl carbon is sp3 hybridized while the aldehyde carbonyl carbon is sp2 hybridized.
E) Once the nucleophile adds to an aldehyde, neither H- nor R- can be eliminated since they are strongly basic.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the product for the reaction. 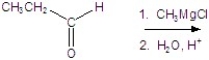
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product of the following reaction? 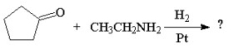
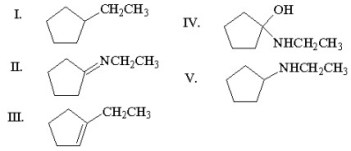
A) I
B) II
C) III
D) IV
E) V
G) D) and E)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
What is the common name for the following compound? 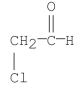
A) chloroaldehyde
B) α-chloroacetaldehyde
C) β-chloroacetaldehyde
D) 2-chloroethanal
E) α-chloroethanal
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the product for the following reaction. 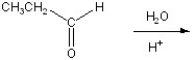
A) ![]()
B) CH3CH2CH2OH
C) CH3CH2CH3
D) ![]()
E) HOCH2CH2CH2OH
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of the imine that results when 4-heptanone is treated with CH3CH2CH2NH2 in the presence of an acid catalyst.
Correct Answer

verified
Correct Answer
verified
Essay
Which carbonyl is more susceptible to nucleophilic attack, that of cyclohexanone or that of hexanal? Provide two reasons for your choice.
Correct Answer

verified
Hexanal. The partial positive charge on ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the major organic product of the following reaction? 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound shown below can form a hemiacetal by reacting with itself in solution. 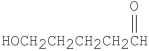 What is the structure of this hemiacetal?
What is the structure of this hemiacetal? 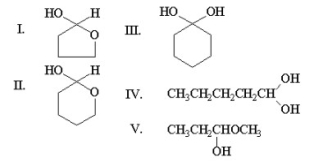
A) I
B) II
C) III
D) IV
E) V
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of cyclohexanone oxime.
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of the diethyl acetal of butanal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is benzaldehyde? 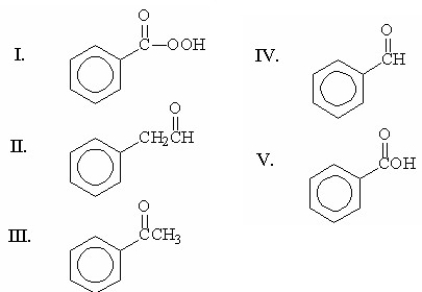
A) I
B) II
C) III
D) IV
E) V
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 116
Related Exams