B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predominant intermolecular force in CH4?
A) London-dispersion forces
B) ion-dipole attraction
C) ionic bonding
D) dipole-dipole attraction
E) hydrogen bonding
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Elemental iodine (I2) is a solid at room temperature.What is the major attractive force that exists among different I2 molecules in the solid?
A) London dispersion forces
B) dipole-dipole interactions
C) ionic-dipole interactions
D) covalent-ionic interactions
E) dipole-dipole attractions
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the figure above,what is the boiling point (°C) of water under an external pressure of 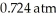 .
.
A) 40
B) 60
C) 70
D) 90
E) 80
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predominant intermolecular force in H2NNH2?
A) hydrogen bonding
B) ion-dipole attraction
C) ionic bonding
D) dipole-dipole attraction
E) London-dispersion forces
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Crystalline solids ________.
A) have their particles arranged randomly
B) have ordered structures
C) are usually very soft
D) exist only at high temperatures
E) exist only at very low temperatures
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following exhibits dipole-dipole attraction between molecules?
A) HCl
B) CF4
C) CS2
D) F2
E) BI3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When KBr dissolves in water,aqueous K+ and Br- ions result.The force of attraction that exists between K+ and H2O is called a(n) ________ interaction.
A) dipole-dipole
B) ion-dipole
C) ion-ion
D) London dispersion force
E) hydrogen bonding
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following,________ is an exothermic process.
A) melting
B) subliming
C) freezing
D) boiling
E) All of the above are exothermic.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species has London dispersion forces as the only intermolecular force?
A) CH3CH2OH
B) Ar
C) NH3
D) HBr
E) H2O
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A supercritical fluid can expand like a ________ to fill a container and has a density similar to that of a ________ so can behave as a solvent.
A) gas, plasma
B) gas, solid
C) solid, gas
D) liquid, gas
E) gas, liquid
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ease with which the charge distribution in a molecule can be distorted by an external electrical field is called the ________.
A) electronegativity
B) hydrogen bonding
C) polarizability
D) volatility
E) viscosity
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As a gaseous element condenses,the atoms become ________ and they have ________ attraction for one another.
A) more separated, more
B) more separated, less
C) closer together, more
D) closer together, less
E) larger, greater
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
________ liquid crystals are colored because the molecular layers are arranged in slightly twisted planes with respect to one another.
A) smectic B
B) cholesteric
C) smectic A
D) smectic C
E) smectic D
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What types of intermolecular forces exist between water and HF?
A) dispersion forces and dipole-dipole forces
B) dispersion forces, dipole-dipole forces, and hydrogen bonds
C) dispersion forces and hydrogen bonds
D) dispersion forces
E) dispersion forces, hydrogen bonds, and ion-dipole forces
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heating curve shown was generated by measuring the heat flow and temperature for a solid as it was heated.The slope of the E-F segment corresponds to the heat capacity of the ________.
A) liquid-gas
B) solid-gas
C) gas
D) solid
E) liquid
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In liquids,the attractive intermolecular forces are ________.
A) very weak compared with kinetic energies of the molecules
B) strong enough to hold molecules relatively close together
C) strong enough to keep the molecules confined to vibrating about their fixed lattice points
D) not strong enough to keep molecules from moving past each other
E) strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the figure above,the boiling point of ethyl alcohol under an external pressure of 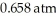 is
is 
A) 20
B) 40
C) 60
D) 80
E) 70
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following substances,only ________ has London dispersion forces as its only intermolecular force.
A) CCl4
B) SnF3
C) CH3OH
D) HI
E) H2O
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the phase diagram shown above,what is the normal melting point (°C) ?
A) 15
B) 25
C) 35
D) 45
E) 55
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 124
Related Exams