A) The potassium used in the softener to replace the calcium and magnesium ions increases the sodium retention in the body.
B) Water softeners work by replacing the calcium and magnesium ions of the water with sodium ions; softened water contains increased levels of sodium ions.
C) The softened water leaches sodium from the body, so a person might not be getting the dietary recommended levels of sodium.
D) A person needs a certain level of calcium and magnesium in the water to help excrete the sodium from their body.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Each circle represents an atom. Which of the following boxes contains an element? A compound? A mixture? 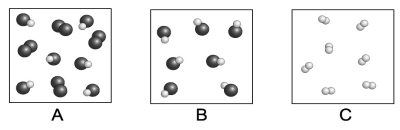
A) element: A, C; compound: A, B, C; mixture: A, B
B) element: C; compound: A, B; mixture: B
C) element: A, C; compound: A, B; mixture: A
D) element: A, C; compound: A, B; mixture: A, B
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Allowing water to cascade or bubble in a fountain during the purification process is an example of ________.
A) a gas dissolving in a liquid
B) a chemical reaction
C) ion exchange
D) a solid dissolving in a liquid
E) only a and b
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the solubility of a compound is 72 grams per liter, how many grams of the compound will dissolve in 0.50 liters?
A) 36 g
B) 72 g
C) 144 g
D) 30 g
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is the solubility of a solid affected by temperature?
A) As temperature goes up, the solubility goes up.
B) As temperature goes down, the solubility goes down.
C) As temperature goes up, the solubility goes down.
D) As temperature goes down, the solubility goes up.
E) both A and B
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why might sodium carbonate (washing soda, Na2CO3) be added to hard water to aid in cleaning?
A) The hard ions in the water are more attracted to the carbonate ions -2 charge.
B) The hard ions are dissolved by the added sodium ions.
C) The soap gets softer due to the added ions.
D) The ions solubilize the soap due to ion-ion intermolecular attraction, which improves the cleaning ability.
E) none of the above
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following picture might best describe a soap or detergent? 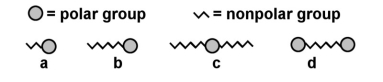
A) a
B) b
C) c
D) d
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which solute graphed above has a solubility in water that does not markedly increase with increasing temperature?
A) NaNO3
B) LiCl
C) KCl
D) NaCl
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Where does most of the solid mass of raw sewage end up after being collected at a treatment facility?
A) It is injected into the water table.
B) It is buried in landfills.
C) It is sold to farmers for fertilizer.
D) It is tapped for the generation of methane gas.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on atomic size, which would you expect to be more soluble in water: helium, He, or nitrogen, N2?
A) Although He is smaller, its outer orbital is filled and the atom will have little attraction to the water molecules.
B) Since He atoms are smaller, more of them can fit into solution, so it has a higher solubility in water.
C) Nitrogen atoms are bigger and so nitrogen molecules should be more soluble in water due to greater dipole-induced dipole attractions.
D) He atoms are bigger and so helium molecules should be more soluble in water due to greater dipole-induced dipole attractions.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A combination of two or more substances in which they no longer retain their chemical properties is called a(n) ________.
A) mixture
B) compound
C) heterogeneous mixture
D) periodic trend
E) suspension
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the difference between a compound and a mixture?
A) They both consist of atoms from different elements.
B) The way in which their atoms are bonded together.
C) One is a solid and the other is a liquid.
D) The components of a mixture are not chemically bonded together.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the first step in treating raw sewage?
A) filtration of solids
B) removal of fine particles by settling
C) removal of grit by settling
D) removal of sludge
E) disinfection
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What makes a semipermeable membrane selective for one chemical species but not another?
A) The pores in the membrane select by size, they are big enough for water only.
B) The material is hydrophilic and therefore only allows water through.
C) The material is hydrophobic and therefore only allows water through.
D) The material chemically reacts with everything but water.
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is most of the energy required for secondary waste water treatment consumed?
A) as electricity to power air pumps for aeration
B) as electricity to illuminate UV lamps
C) as heat to run distillation equipment
D) as electricity to power hydraulic pumps for reverse osmosis
E) none of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Classify the following as element, compound, or mixture, and justify your classifications: table salt, stainless steel, table sugar, aluminum, ice.
A) mixture; element; compound; element; element
B) compound; mixture; compound element; compound
C) mixture; compound; mixture; element; compound
D) compound; element; compound; element; compound
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When blue food coloring is stirred into water, the result is a ________.
A) homogeneous mixture called a solution
B) homogeneous mixture called a suspension
C) heterogeneous mixture called a solution
D) heterogeneous mixture called a suspension
E) pure liquid
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of water are there in 100. grams of water?
A) 1800 moles
B) 100 moles
C) 0.018 moles
D) 5.55 moles
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of water that is 99.9999 percent pure contains 0.0001 percent impurities. Consider from Chapter 1 that a glass of water contains on the order of a trillion trillion (1 × 1024) molecules. If 0.0001 percent of these molecules were the molecules of some impurity, about how many impurity molecules would this be?
A) 1000 (one thousand: 1 × 103)
B) 1,000,000 (one million: 1 × 106)
C) 1,000,000,000 (one billion: 1 × 109)
D) 1,000,000,000,000,000,000 (one million trillion: 1 × 1018)
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does the purchase and use of a home beverage carbonator help to minimize carbon dioxide emissions?
A) It allows the consumer to avoid using commercial sodas that require much gasoline for shipping.
B) Home carbonators are more efficient at forcing the carbon dioxide into solution.
C) Industrial carbonators are driven by mechanical pumps that require large amounts of gasoline in order to operate.
D) False. Home carbonators have the net effect of producing more carbon dioxide per liter of carbonate water.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 141
Related Exams