A) n-type silicon
B) p-type silicon
C) an excess of negative charges
D) electrons that are free to roam
E) A and C
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Electrolysis is an example of ________.
A) a physical change
B) a chemical change
C) an acid-base reaction
D) an exothermic reaction
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is the junction barrier between n-type and p-type silicon much like a one-way valve?
A) The electrons can only be lost from the p-type silicon wafer.
B) The orientation of the charge around the barrier allows the electrons to flow in only one direction.
C) The electrons can only be lost from the n-type silicon wafer.
D) Only one side of the barrier is exposed to light, which causes the electrons to flow in the opposite direction.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following set of redox reactions takes place as shown. Container A Container B Fe→ Fe2+ + 2e- Cu2+ + 2e- → Cu If you had two containers filled with the ion solution described above with only a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB) , describe what happens.
A) A little bit of iron is converted into iron ions and a little bit of copper ion is converted into copper metal.
B) All of the iron is transformed into iron ions and all of the copper is transformed into copper ions.
C) All of the iron is transformed into iron ions and all of the copper ions are transformed into copper metal.
D) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper ions.
E) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper metal.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following materials is most likely to undergo reduction?
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an operating photovoltaic cell, electrons move through the external circuit to the negatively charged p-type silicon wafer. How can the electrons move to the negatively charged silicon when they themselves are negatively charged? Don't like signs repel each other?
A) This is a technological hurdle that has greatly inhibited the advancement of photovoltaic technology.
B) An electric current is possible because the energy from the sun momentarily reverses the charge of the electrons, which are then called positrons (p-type) .
C) The p-type silicon wafer is actually positively charged.
D) The energy of the sunlight knocks the electrons into a direction that they would otherwise not go.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical equation for the combustion of propane, C3H8, is shown below. Through this reaction is the carbon oxidized or reduced? 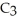
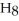 + 5
+ 5 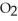 → 3
→ 3 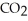 + 4
+ 4 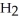 O
O
A) The carbon is oxidized.
B) The carbon is reduced.
C) Some of the carbons are oxidized while others are reduced.
D) The carbon is neither oxidized nor reduced.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements describes what happens when you press a n-type silicon wafer against a p-type silicon wafer?
A) The n-type wafer becomes slightly positive, while the p-type becomes slightly negative.
B) The p-type wafer becomes slightly positive, while the n-type becomes slightly negative.
C) There is no change.
D) They both become slightly negative.
E) They both become slightly positive.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following materials is most likely to undergo oxidation?
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B
G) A) and E)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Chemical equations need to be balanced not only in terms of the number of atoms, but also by the charge. In other words, just as there should be the same number of atoms before and after the arrow of an equation, there should be the same charge. What set of coefficients is necessary to balance the following chemical equation? ____ 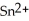 + ____ Ag → ____ Sn + ____ Ag⁺
+ ____ Ag → ____ Sn + ____ Ag⁺
A) 1, 2, 1, 2
B) 1, 1, 1, 2
C) 1, 2, 2, 2
D) 1, 1, 2, 1
F) C) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
How might electrolysis be used to raise the hull of a sunken ship?
A) An electric current passed through the hull of the ship would produce electrolysis and the gases trapped in the compartments of the vessel would push it upwards.
B) The electrolysis of the water beneath the hull of the ship would boil the water and create upward pressure, thus buoying the ship upward.
C) The gaseous products of the electrolysis of water could be collected with large bags attached to the hull of the ship. As the bags inflate with the gas they are buoyed upward pulling the ship also upward.
D) Electrolysis could only be used to raise the hull of a ship if the ship were made of iron. If so, the electrolysis of the iron metal might produce enough gas to lift the ship.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iron is useful for reducing copper ions to copper metal. Might it also be used to reduce sodium ions to sodium metal?
A) No, because sodium ions are too difficult to reduce.
B) Yes, especially in that sodium has such a low ionization energy.
C) No, because the sodium ions in turn oxidize the iron.
D) Yes, especially in that sodium has such a high ionization energy.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why are combustion reactions generally exothermic?
A) Hydrogen easily pulls electrons from the oxygen in water molecules and are reduced.
B) During combustion oxygen is oxidized, which gives off lots of energy.
C) Combustion involves the transfer of electrons to oxygen, which has an extremely high tendency for gaining electrons.
D) It is highly exothermic because combustion involves an oxidation-reduction reaction between a metallic material and oxygen.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When you have p-type silicon and n-type silicon joined together, why don't electrons dislodged by light in the n-type cross the junction barrier and enter the p-type?
A) The junction barrier is unidirectional. Electrons can only move from the p-type to the n-type silicon through the junction.
B) The photoelectric effect causes a charge buildup which prevents the flow of electrons in either direction across the junction barrier.
C) The boron in p-type silicon's crystal lattice must maintain its "electron holes" as bonding sites. This prevents the electrons dislodged by light in n-type silicon from crossing the junction barrier.
D) The arsenic in n-type silicon has an extra valence electron whereas the boron in p-type silicon is missing one. This difference inhibits the electrons dislodged by light in the n-type from crossing the junction barrier.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is one of the advantages of a fuel cell?
A) They can run for a very long time as long as you keep adding fuel.
B) They are more efficient than combustion.
C) They have lower emission of pollutants than combustion.
D) all of the above
E) only A and B
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does n-type silicon differ from pure silicon?
A) There are other elements with more valence electrons than silicon in the silicon matrix.
B) There are extra negative charges.
C) There are no negative charges.
D) There are other elements with less valence electrons than silicon in the silicon matrix.
E) There are missing silicon atoms resulting in vacancies in the silicon matrix.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the half-reactions below would be complementary, balance the following half-reaction, and be chemically reasonable? Br2 → 2 Br-
A) 2 K → 2 K+
B) K+ → K
C) K → K+
D) 2 K+ → 2 K
E) K → K2+
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is one of the drawbacks to using fuel cells?
A) The fuel of the fuel cells is hard to handle.
B) They are less efficient than combustion energy sources or batteries.
C) They are very big and bulky, which makes them hard to use in small devices.
D) There are no drawbacks.
E) only A and B
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oxidation of iron to rust is a problem structural engineers need to be concerned about, but the oxidation of aluminum to aluminum oxide is not. Why?
A) Since aluminum is so light and inexpensive, it is easily replaced when corroded.
B) Aluminum oxide is insoluble in water and thus forms a protective coating that prevents continued oxidation of the aluminum.
C) Aluminum oxide is even stronger than aluminum.
D) The oxidation of aluminum is a very slow reaction, taking hundreds of years before any structural changes are noticed.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are gained or lost in the following half-reaction? Na → Na+
A) 1 electron is lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 1 electron is gained
E) 2 electrons are gained
G) B) and E)
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 138
Related Exams