A) 14.8 L
B) 105 L
C) 3.82 L
D) 22.4 L
E) 27.1 L
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 0.334 g sample of an unknown halogen occupies 109 mL at 398 K and 1.43 bar.What is the identity of the halogen?
A) Br2
B) F2
C) Cl2
D) I2
E) At2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the graph below,determine the gas that has the lowest density at STP. 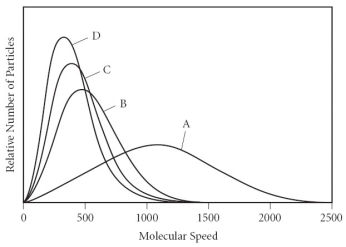
A) A
B) B
C) C
D) D
E) All of the gases have the same density at STP.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If NO2 and NH3 are allowed to effuse through a porous membrane under identical conditions,the rate of effusion for NH3 will be ________ times that of NO2.
A) 0.37
B) 0.61
C) 1.6
D) 2.7
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the temperature,in K,of 2.20 moles of gas occupying 3.50 L at 3.30 bar.
A) 63.1 K
B) 5.25 K
C) 337 K
D) 28.0 K
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE?
A) At a given temperature,lighter gas particles travel more slowly than heavier gas particles.
B) The smaller a gas particle,the slower it will effuse.
C) The higher the temperature,the lower the average kinetic energy of the sample.
D) At low temperatures,intermolecular forces become important and the pressure of a gas will be lower than predicted by the ideal gas law.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of 0.300 moles of nitrogen occupies 0.600 L.Under the same conditions,what number of moles occupies 1.200 L?
A) 0.600 moles
B) 1.50 moles
C) 0.33 moles
D) 6.00 moles
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of CO2 are contained in a 10.0 L tank at 7.53 bar and 485 K?
A) 1.89 × 1024 molecules
B) 1.12 × 1024 molecules
C) 8.32 × 1024 molecules
D) 4.89 × 1024 molecules
E) 3.63 × 1024 molecules
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of N2 are in a 400.0 mL container at 1.0399 bar and 135 °C?
A) 7.01 × 1021 molecules
B) 7.38 × 1021 molecules
C) 2.12 × 1022 molecules
D) 2.23 × 1022 molecules
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the volume of SO2 (at STP) formed from the reaction of 96.7 g of FeS2 and 55.0 L of O2 (at 398 K and 1.20 bar) .The molar mass of FeS2 is 119.99 g mol-1. 4FeS2(s) + 11O2(g) → 2Fe2O3(s) + 8SO2(g)
A) 36.1 L
B) 45.3 L
C) 18.1 L
D) 27.6 L
E) 32.5 L
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume will 4.91 × 1022 atoms of Ne occupy at STP?
A) 1.10 L
B) 2.00 L
C) 2.24 L
D) 3.11 L
E) 1.85 L
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Define pressure.
Correct Answer

verified
Pressure is the force exerted ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following would have a density of 1.37 g L-1 at 7.0 °C and 0.9967 bar?
A) N2
B) O2
C) Kr
D) Rn
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the gas particle that travels the fastest.
A) H2
B) O2
C) Ne
D) N2
E) CO
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many litres of oxygen are needed to exactly react with 19.8 g of methane at STP? CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
A) 13.9 L
B) 27.8 L
C) 56.0 L
D) 60.5 L
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Explain what the term "mean free path" describes.How does it change with decreasing pressure?
Correct Answer

verified
Mean free path describes the distance th...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
You have three cylinders containing O2 gas at the same volume and pressure.Cylinder A is at -15 °C,cylinder B is at -5 °F,cylinder C is at 255 K.Which cylinder contains the largest mass of oxygen?
A) cylinder A
B) cylinder B
C) cylinder C
D) All cylinders contain the same mass of O2.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unknown gas has a root mean square velocity of 332 m s-1 at 313 K.Identify the unknown gas.
A) Kr
B) O2
C) Ar
D) F2
E) Cl2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of NO2 is contained in a 13.0 L tank at 4.58 bar and 385 K?
A) 18.8 g
B) 53.1 g
C) 24.4 g
D) 85.6 g
E) 69.2 g
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 14.0 g of zinc metal reacts with excess HCl,how many litres of H2 gas are produced at STP?
A) 0.208 L
B) 0.416 L
C) 4.86 L
D) 9.60 L
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 146
Related Exams