B) False
Correct Answer

verified
Correct Answer
verified
True/False
The molecular formula is equal to the empirical formula multiplied by a whole number integer.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many hydrogen atoms are in 35.0 grams of hydrogen gas?
A) 4.25 × 1025
B) 2.09 × 1025
C) 2.12 × 1025
D) 1.05 × 1025
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The simplest formula for hydrogen peroxide is HO.To determine its molecular formula,it is necessary to know
A) the properties of hydrogen peroxide.
B) the density of hydrogen peroxide.
C) the molar mass of hydrogen peroxide.
D) the number of moles of hydrogen peroxide in 1.00 g of the substance.
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The correct formula for calculating mass percent of X in compound XY is: 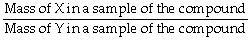 = Mass % X
= Mass % X
B) False
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
What is the molecular formula of a compound given the molar mass of the compound is 186.5 grams and the empirical formula is C2H7?
A) C4H14
B) C3H21
C) C2H7
D) C2H14
E) none of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that sodium chloride is 39.0% sodium by mass,how many grams of sodium chloride are needed to have 750.mg of Na present?
A) 1.92
B) 0.293
C) 1,920
D) 29.3
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chromium oxide compound contains 104.0 grams of chromium and 48.0 grams of oxygen.What is the most likely empirical formula of this compound?
A) CrO
B) CrO2
C) CrO3
D) Cr2O3
E) Cr3O2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the empirical formula of a compound containing 60.3% magnesium and 39.7% oxygen.
A) MgO
B) MgO2
C) Mg2O3
D) Mg2O
E) none of the above
G) A) and C)
Correct Answer

verified
A
Correct Answer
verified
True/False
The number 6.022 × 1023 is six times larger than the number 6.022 × 1022.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of n when the empirical formula is C3H5 and the molecular mass is 205.4 g/mol?
A) 0.02
B) 5
C) 10
D) 140
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of sulfur trioxide are in 78.0 grams?
A) 5.87 × 1023
B) 7.33 × 1023
C) 3.76 × 1027
D) 0.974
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The numerical value of the mole is defined as being equal to the number of atoms in exactly 12 grams of pure carbon-12.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Bauxite is an ore that contains the element aluminum.If you obtained 108 grams of aluminum from an ore sample that initially weighed 204 grams,what is the mass percent of aluminum in this bauxite ore?
A) 52.9
B) 15.6
C) 0.53
D) 47.1
E) none of the above
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
A molecule that has an empirical formula of HO and a molar mass of 34.02 gram must have a molecular formula of H2O2.
B) False
Correct Answer

verified
True
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest mass percent of "O"?
A) MnO
B) MnO2
C) Mn2O3
D) Mn3O2
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 500.gram iron ore sample was determined to contain 242 grams of iron.What is the mass percent of iron in the ore?
A) 93.7
B) 48.4
C) 51.6
D) 32.6
E) none of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass percent of carbon in oxalic acid,H2C2O4?
A) 2.24
B) 13.3
C) 26.7
D) 34.5
E) none of the above
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms are in 5.80 moles of He?
A) 6.02 × 1023
B) 1.03 × 1023
C) 4.00
D) 3.49 × 1024
E) none of the above
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the empirical formula of a compound containing 83% potassium and 17.0% oxygen.
A) KO
B) KO2
C) K2O3
D) K2O
E) none of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 111
Related Exams