A) When the temperature of two low density gases,which are initially at the same temperature,pressure and volume,is increased by the same amount,the volume increases by the same amount.
B) When the volume of two low density gases,which are initially at the same temperature,pressure and volume,is compressed by the same amount,the temperature increases by the same amount.
C) A P-T plot of any gas at low density intercept the T-axis at the same point.
D) The intercept at the ordinate for PV/nT vs P plot of any gas is the gas constant.
E) The ideal gas law is valid for high density of all gases.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A temperature difference of 9ºF is the same as a difference of
A) 5ºC
B) 9ºC
C) 20ºC
D) 68ºC
E) 100ºC
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the absolute temperature of a gas is doubled,what is the change in the average kinetic energy of its molecules?
A) no change
B) increases by a factor of 2
C) decreases by a factor of 2
D) increases by a factor of ![]()
E) decreases by a factor of ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Doubling the Kelvin temperature of a gas increases which of the following measures of its molecular velocity by a factor of 1.4?
A) the rms speed
B) the average speed
C) the most probable speed
D) All three of these speeds are correct.
E) None of these is correct.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the question: 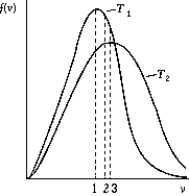 -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
A) The curve labeled T1 represents the distribution for the higher temperature molecules.
B) The point labeled "1" corresponds to the rms speed of the molecules whose temperature is T1.
C) The point labeled "2" corresponds to the maximum speed of the molecules whose temperature is T1.
D) The point labeled "3" corresponds to the rms speed of the molecules whose temperature is T1.
E) None of these is correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Normal human body temperature is 98.6ºF.What is the corresponding Celsius temperature?
A) 54.8ºC
B) 72.6ºC
C) 40.0ºC
D) 37.0ºC
E) 35.5ºC
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A constant-volume gas thermometer reads 6.66 kPa at the triple point of water.What is the pressure reading at the normal boiling point of water?
A) 2.44 kPa
B) 18.2 kPa
C) 9.10 kPa
D) 11.8 kPa
E) 4.87 kPa
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On the basis of the kinetic theory of gases,when the absolute temperature is doubled,the average kinetic energy of the gas molecules changes by a factor of
A) 16
B) 2
C) ![]()
D) 4
E) 0.5
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A device used to measure the speed,v,of molecules is shown below.When the cylinder is rotated,only molecules that are of the right speed will pass through the slanted slot and be pickup by the detector.Drive an expression for the speed v in terms of the angular speed, , ,and L.L is the length of the cylinder and is the angle subtended by the slanted slot. 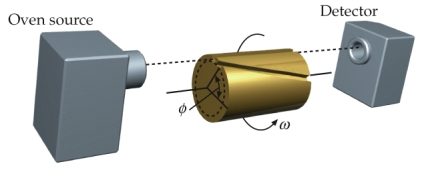
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you plot a graph with Fahrenheit temperatures along the horizontal axis and the corresponding Celsius temperatures along the vertical axis,the slope of the equal-temperature line will be
A) -40 Fº/Cº
B) 32 Fº/Cº
C) 1.8 Fº/Cº
D) 0.56 Fº/Cº
E) 0 Fº/Cº
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the question:  -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
A) The curve labeled T2 represents the distribution for the higher temperature molecules.
B) The point labeled "1" corresponds to the rms speed of the molecules whose temperature is T1.
C) The point labeled "2" corresponds to the maximum speed of the molecules whose temperature is T1.
D) The point labeled "3" corresponds to the average speed of the molecules whose temperature is T1.
E) None of these is correct.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A temperature difference of 5 Cº is the same as a difference of
A) 41 Fº
B) 14 Fº
C) 9 Fº
D) 5 Fº
E) -15 Fº
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a vacuum system,a container is pumped down to a pressure of 1.33 10-6 Pa at 20ºC.How many molecules of gas are there in 1 cm3 of this container? (Boltzmann's constant k = 1.38 10-23 J/K)
A) 3.3 108
B) 4.8 109
C) 3.3 1014
D) 7.9 1012
E) 4.8 1012
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rms speed of oxygen molecules is 460 m/s at 0ºC.The molecular weight of oxygen is 8 times the molecular weight of helium.The rms speed of helium at 40ºC is approximately
A) 3.68 km/s
B) 1.84 km/s
C) 1.40 km/s
D) 880 m/s
E) 440 m/s
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At what common Celsius temperature is the rms velocity of oxygen molecules (molar mass = 32 g/mol) double that of hydrogen molecules (molar mass = 2.0 g/mol) ?
A) -50
B) zero
C) no such temperature exists
D) 2.0
E) 16
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the question:  -The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
-The figure shows the distribution of the molecular speeds of a gas for two different temperatures.
A) The curve labeled T1 represents the distribution for the higher temperature molecules.
B) The point labeled "1" corresponds to the rms speed of the molecules whose temperature is T1.
C) The point labeled "2" corresponds to the maximum speed of the molecules whose temperature is T1.
D) The point labeled "3" corresponds to the average speed of the molecules whose temperature is T1.
E) None of these is correct.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If both the temperature and the volume of an ideal gas are doubled,the pressure is
A) increased by a factor of 4.
B) doubled also.
C) unchanged.
D) diminished by a factor of ¼.
E) None of these is correct.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Boltzmann's constant,k,has a value of 1.381 10-23 J/K.What is the significance of the constant?
A) It defines a characteristic energy at the microscopic level given the temperature in kelvins.
B) It allows the pressure of the gas to be calculated given the volume and temperature.
C) It measures the average kinetic energy of a molecule at a given temperature for each degree of freedom.
D) (A) and (B)
E) (A) and (C)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A temperature of 14ºF is equivalent to
A) -10ºC
B) 7.77ºC
C) 25.5ºC
D) 26.7ºC
E) 47.7ºC
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A thermometer is constructed by filling a small glass tube with a liquid that expands linearly with temperature.The thermometer is then calibrated at 0 C and 100 C,and the scale evenly divided between the two values.Unfortunately a manufacturing defect results in the middle one-third of the tube being narrower,otherwise the tube has uniform diameter.For what range of temperatures is the reading accurate? 
A) 0 C to 33 C
B) 0 C and 67 C
C) 0 C to 33 C and 67 C to 100 C
D) 67 C to 100 C
E) At no point does this thermometer gives the right reading except at 0 C and 100 C.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 78
Related Exams