B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is identical to the one shown below? 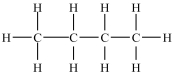
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pairs of compounds are constitutional isomers?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The molecules below are constitutional isomers. 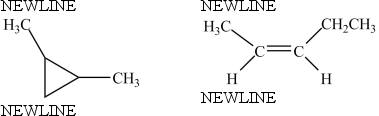
B) False
Correct Answer

verified
Correct Answer
verified
True/False
CO2 is a greenhouse gas.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the classification of each labeled carbon atom in this structure? 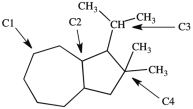
A) C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 4°.
B) C1 is a 2°, C2 is 3°, C3 is a 3°, and C4 is 4°.
C) C1 is a 1°, C2 is 2°, C3 is a 3°, and C4 is 1°.
D) C1 is a 2°, C2 is 3°, C3 is a 2°, and C4 is 3°.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the classification of the carbon atom that bonded to the -OH group in menthol? 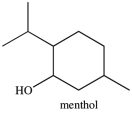
A) primary
B) secondary
C) tertiary
D) quaternary
F) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The compounds below are identical. 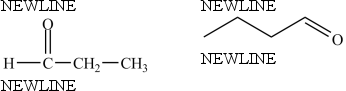
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many secondary carbons are in the straight-chain alkane with the formula C4H10?
A) 0
B) 1
C) 2
D) 3
E) 4
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound is a constitutional isomer of the one shown below? 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) More than one of the compounds above is a constitutional isomer.
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
Oxidation results in a decrease in the number of C-O bonds and an increase in the number of C-H bonds.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The compounds below are constitutional isomers. 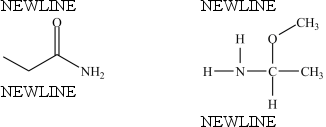
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The IUPAC name of the molecule (CH3)2CH(CH2)2CH3 is 2-methylhexane.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The compounds below are constitutional isomers. 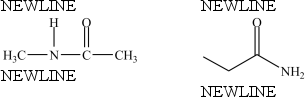
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of this compound? 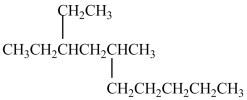
A) 3-ethyl-5-methyldecane
B) 3-ethyl-2-pentylhexane
C) 3-ethyl-5-pentylhexane
D) 8-ethyl-6-methyldecane
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The compounds below are constitutional isomers. 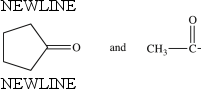
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
There are _____ carbons in the longest continuous chain of carbons in the molecule below. 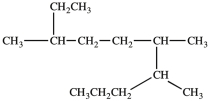
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a constitutional isomer of the compound shown below? 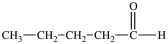
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) More than one of the molecules above is a constitutional isomer of the original molecule.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
There are _____ carbons in the longest continuous chain of carbons in the molecule below. 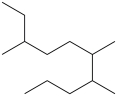
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of this compound? 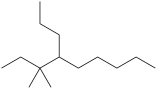
A) 4-isopentylnonane
B) 3-ethyl-4-propylnonane
C) 3, 3-dimethyl-4-propylnonane
D) 3, 3-dimethyl-4-butylnonane
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 106
Related Exams