A) -2
B) +2
C) +6
D) +4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which example BEST represents the reaction between copper and hydrochloric acid?
A) Cu + 2 HCl CuCl2 + H2
B) Cu + 2 HCl CuH2 + Cl2
C) Cu + H2Cl CuCl + H2
D) Copper and hydrochloric acid do not react.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is FALSE?
A) Silver ion (Ag+) to silver atom is reduction.
B) Chloride (Cl-) ion to chlorine atom is reduction.
C) Hydrogen atom to hydride ion (H-) is reduction.
D) Sulfur atom to sulfide ion (S2-) is reduction.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell,use the partial activity series included to identify the components labeled 1,2,3,and 4 respectively. 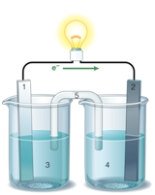 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) iron,nickel,iron ion solution,nickel ion solution
B) nickel,iron,iron ion solution,nickel ion solution
C) nickel,iron,nickel ion solution,iron ion solution
D) iron,nickel,nickel ion solution,iron ion solution
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which example represents the reduction half-reaction for cobalt/cobalt(II) ?
A) Co Co2+ + 2 e-
B) Co + 2 e- Co2-
C) Co2+ + 2 e- Co
D) Co2+ Co + 2 e-
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of carbon in carbon dioxide,CO2?
A) -2
B) +2
C) +6
D) +4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the accompanying partial activity series,which element(s) will react with acid? Zn Zn2+ + 2e- Fe Fe2+ + 2e- Ni Ni2+ + 2e- H2 2H+ + 2e- Cu Cu2+ + 2e- Ag Ag+ + e-
A) nickel
B) copper
C) silver
D) nickel,copper,and silver
E) None of these elements will react with acid.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider an electrochemical cell consisting of zinc and cobalt half-cells.Use the partial activity series included to determine the direction in which the electrons in the wire will flow. Zn Zn2+ + 2e- Fe Fe2+ + 2e- Co Co2+ + 2e- Ni Ni2+ + 2e- Cu Cu2+ + 2e-
A) from the zinc anode to the cobalt cathode
B) from the zinc cathode to the cobalt anode
C) from the cobalt anode to the zinc cathode
D) from the cobalt cathode to the zinc anode
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the balanced net ionic equation for the reaction consisting of the two half-reactions given. Mg Mg2+ + 2 e- Pb4+ + 4 e- Pb
A) Pb (s) + Mg2+ (aq) Pb4+ (aq) + Mg (s)
B) Mg (s) + Pb4+ (aq) Pb (s) + Mg2+ (aq)
C) 2 Mg (s) + Pb4+ (aq) Pb (s) + 2 Mg2+ (aq)
D) Pb (s) + 2 Mg2+ (aq) Pb4+ (aq) + 2 Mg (s)
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which example illustrates a redox reaction?
A) metal and nonmetal
B) double displacement
C) neutralization
D) precipitation
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the partial activity series included to determine which reaction will NOT occur spontaneously. Zn Zn2+ + 2e- Fe Fe2+ + 2e- Co Co2+ + 2e- Ni Ni2+ + 2e- Cu Cu2+ + 2e-
A) Fe (s) + Co2+ (aq) Fe2+ (aq) + Co (s)
B) Zn (s) + Ni2+ (aq) Zn2+ (aq) + Ni (s)
C) Co (s) + Fe2+ (aq) Co2+ (aq) + Fe (s)
D) Ni (s) + Cu2+ (aq) Ni2+ (aq) + Cu (s)
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following net ionic equation,select the CORRECT pairing of half-reactions. 2 Al (s) + 3 Pb2+ (aq) 2 Al3+ (aq) + 3 Pb (s)
A) reduction: Al Al3+ + 3 e-; oxidation: Pb2+ + 2 e- Pb
B) reduction: Pb2+ + 2 e- Pb; oxidation: Al Al3+ + 3 e-
C) reduction: Al3+ + 3 e- Al; oxidation: Pb2+ + 2 e- Pb
D) reduction: Pb Pb2+ + 2 e-; oxidation: Al Al3+ + 3 e-
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 52 of 52
Related Exams